Conjugation Technology
Conjugation drugs (XDC Drug) development represents a niche track of custom manufacturing. While antibodies, linkers, and drugs are important, the real core barriers and protective moats stem from the design of conjugations, which rely on the core technical staff's understanding of drug-likeness and inspiration. As a critical component that determines the systemic toxicity and clinical efficacy of conjugated drugs, the role of conjugation technology is primarily manifested in two aspects:
(1)1.Ensuring that when the conjugated drug remains in the bloodstream, the cytotoxin is tightly connected to the monoclonal antibody. Unstable linkers in the blood can lead to premature release of cytotoxins, causing severe systemic toxic side effects and reducing the number of conjugated drugs reaching tumor tissues.
(2)2.Ensuring that when the conjugated drug enters tumor cells, the cytotoxin can be effectively released. It is evident that conjugation technology is a key component in determining the systemic toxicity and clinical efficacy of conjugated drugs. The design and development of conjugated drugs are not simply a combination of antibodies/peptides, conjugation technology, and cytotoxins/ligands, but require the selection of appropriate combinations to optimize overall efficacy and safety. The production process is complex, involving multiple production stages such as monoclonal antibodies, bulk solutions, and formulations, especially in biologic conjugation technology, which is highly challenging and difficult. This poses significant challenges to the research, development, production, and commercialization capabilities of original drug companies.
Overall, the antibodies/peptides and drugs for conjugated drugs can be purchased from the market or custom synthesized, but assembling them into an effective conjugated drug requires highly flexible conjugation processes (connection points, drug loading, spatial distance between antibodies and drugs, stability, etc.) to potentially succeed in drug commercialization.

Technical Features of OPA LinkTech™
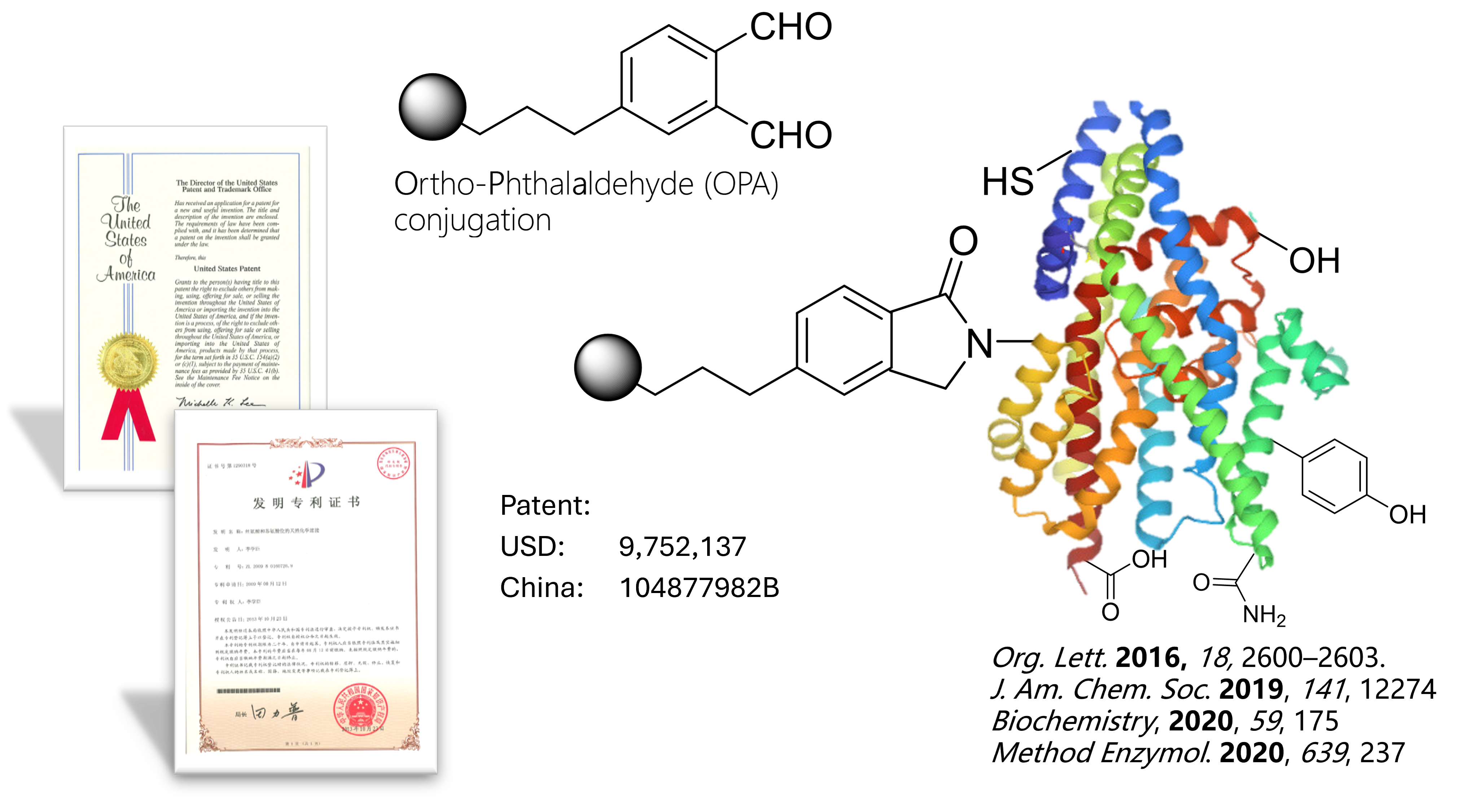
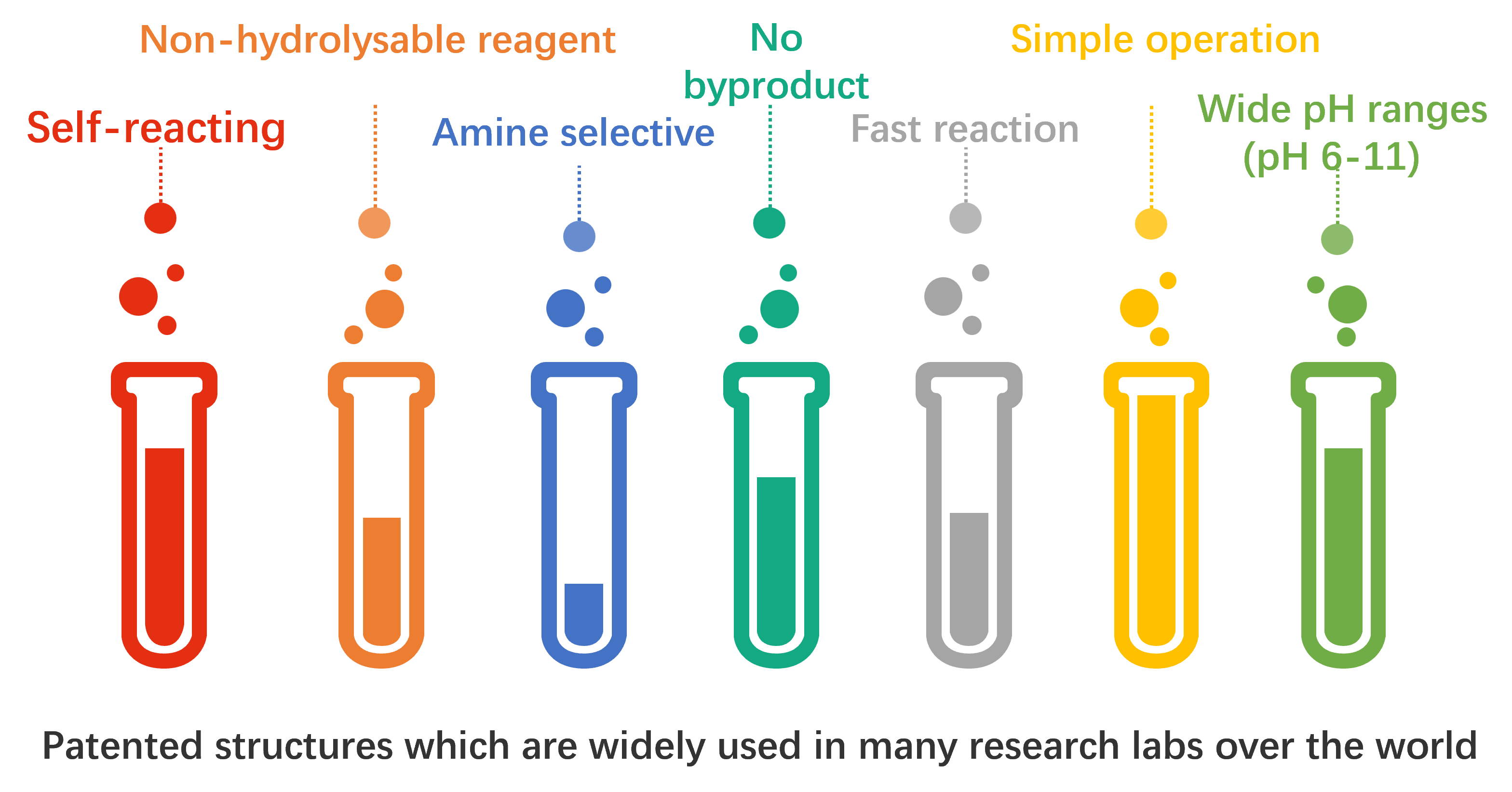
Serilink’s innovative conjugation technology, OPA LinkTech™, is a novel and efficient peptide/protein conjugation technology that operates through amine reactions. This technology features rapid reaction rates (4.3 M-1 s-1), flexible reaction conditions, no side effects, and simple operation.
Based on OPA LinkTech™, a comprehensive platform development system has been established, enabling large-scale, stable preparation of various plug-and-play OPA reagent kit products, including OPA-fluorescence, OPA-PEG, OPA-toxin, OPA-polymer, OPA-Click chemistry, and more.
Compared to traditional techniques, this linkage method does not compromise the pharmacological efficacy of conjugated drugs, while offering more flexible reaction conditions, linker fine-tuning, and more stable product forms. Therefore, OPA LinkTech™ can be extensively applied in the research and development of conjugated drugs such as ADCs (Antibody-Drug Conjugates), ARCs (Antibody-Radioligand Conjugates), and PDCs (Peptide-Drug Conjugates). Currently, Serilink has successfully developed a series of OPA reagent kits, providing efficient services for pharmaceutical companies developing various types of conjugated drugs.